



Indications: Chronic Hepatitis B (CHB)
Status: Preclinical
SP-2021A is a human anti-CD33 monoclonal antibody developed by LectinStars Pharmaceuticals Ltd. In recent years, the global threat posed by Hepatitis B virus infection has escalated, presenting a significant challenge to public health worldwide. According to the World Health Organization's 2019 report, approximately 296 million people globally are infected with the Hepatitis B virus, resulting in around 820,000 deaths annually due to complications such as liver cirrhosis and hepatocellular carcinoma triggered by the infection. Currently, mainstream medications for treating chronic B-type Hepatitis primarily focus on viral replication and oral drugs that slow liver inflammation. However, these medications fall short of providing a complete cure for chronic Hepatitis B.
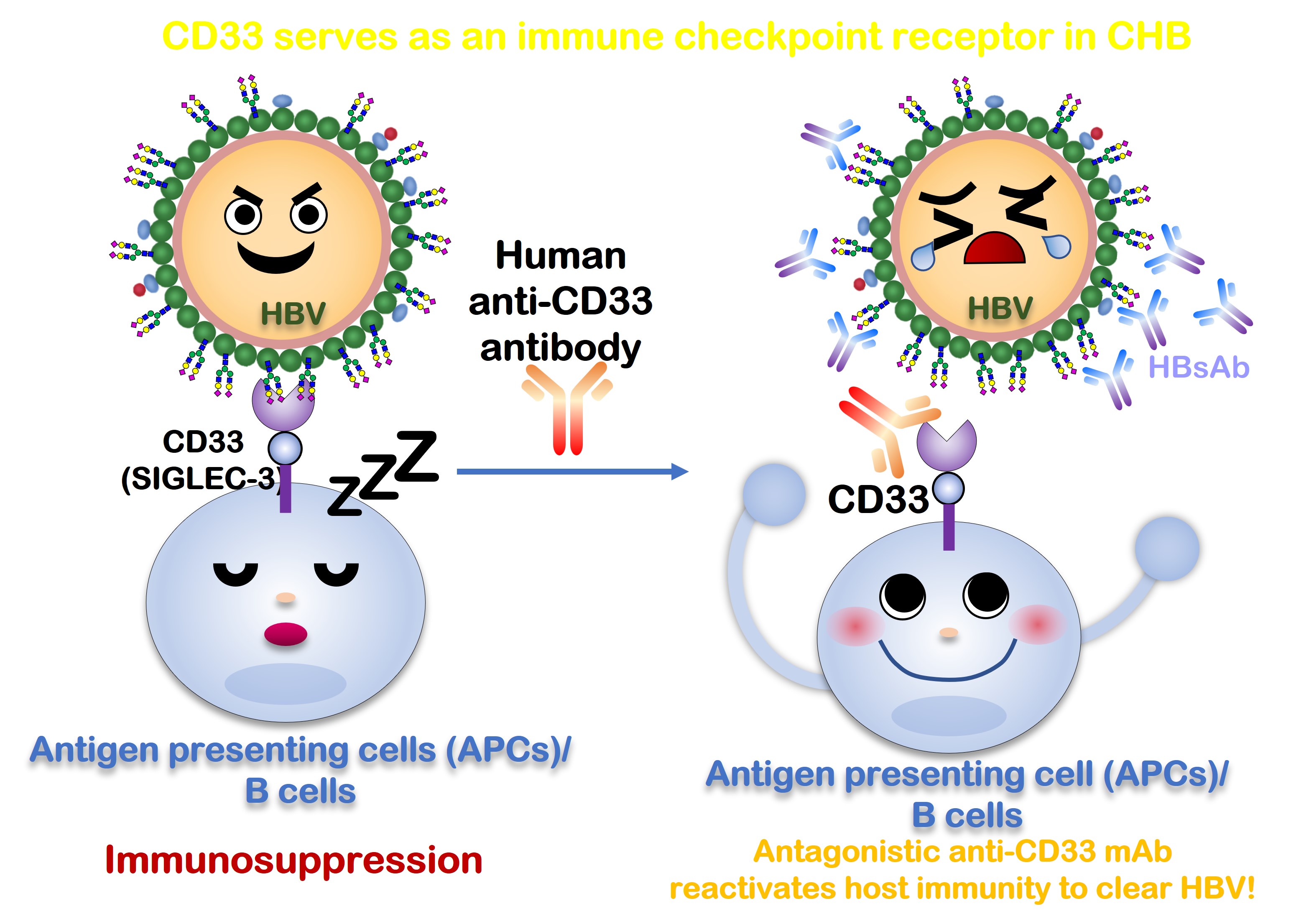
SP-2021A is a monoclonal antibody drug targeting the immune checkpoint CD33 (Siglec-3) on human immune cells. Through validation in in vitro experiments, we have observed that SP-2021A can effectively block the binding of sialoglycans on the surface antigen (HBsAg) of the Hepatitis B virus. This prevents the suppression of the immune system, allowing the patient's body to generate antibodies against the Hepatitis B virus, thereby achieving a curative effect. As a groundbreaking therapeutic, SP-2021A presents a new treatment avenue for individuals with chronic Hepatitis B, also offering substantial therapeutic promise and the potential to transform the current treatment paradigm for this condition. Anticipating further clinical trials to validate its effectiveness in clinical practice.